Hexagonal Boron Nitride VS. Cubic Boron Nitride

Boron nitride ceramic is a thermally and chemically stable refractory material made up of one molecule each of boron and nitrogen. The substance is available in either solid or powder form. It also occurs in three main crystalline forms, known as hexagonal boron nitride (h-BN), cubic boron nitride (c-BN), and wurtzite boron nitride (w-BN). Hexagonal BN is the most stable and soft form, which is why it is used as a lubricant and additive to cosmetic products. There are other polymorphic but less common forms, such as a-BN, t-BN, r-BN, m-BN, and o-BN. Cubic boron nitride (c-BN), which is the hardest form of the compound, is analogous to diamond and is mostly applied in various processes as an abrasive. The wurtzite boron nitride (w-BN) is quite rare but similar to lonsdaleite. It is even believed to be harder than c-BN. Another thing worth noting about boron nitride is that it does not occur naturally. It is mostly obtained as a synthetic substance. This post compares the synthesis, properties, and applications of hexagonal boron nitride vs. cubic boron nitride.
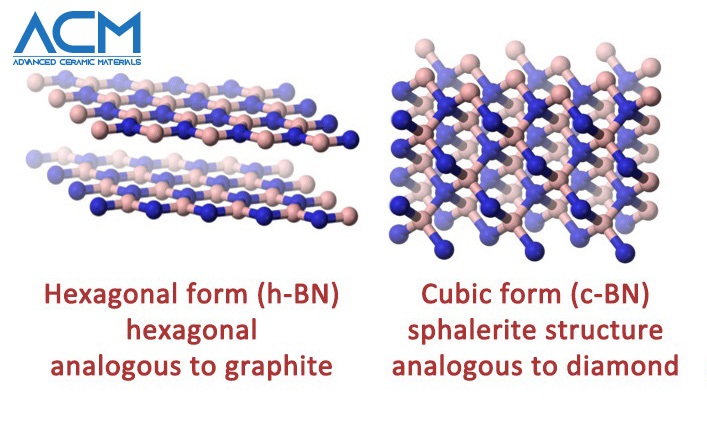
Hexagonal boron nitride vs. cubic boron nitride
Hexagonal Boron Nitride
Hexagonal boron nitride is synthesized by nitridation or ammonolysis of boric oxide at a very high temperature. Hexagonal BN has a microstructure similar to graphite. The plate-like microstructure and layered lattice structure make it a good lubricating substance. It is resistant to sintering and is mostly formed by hot pressing. Structurally, h-BN is a white slippery powder; in another way analogous to graphite. The size of the h-BN powder flake available commercially varies from hundreds of nanometers to tens of microns. The h-BN crystal structure is hexagonal and the BN molecule is partially ionic, which reduces the conductance capacity of electricity. Both h-BN and c-BN exhibit high chemical and thermal stability. But h-BN is more stable than c-BN even without deteriorating at temperatures as high as 1000°C (in air), 1400°C (in vacuum), and 2850°C (in inert atmosphere). Hence, h-BN has thermal conductivity values close to graphene, which is why it is considered one of the most advanced ceramic materials today.
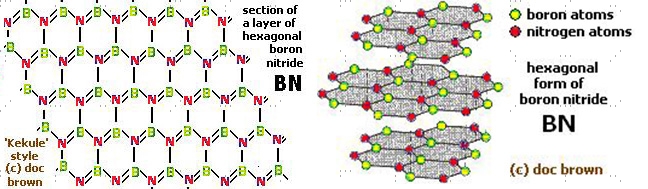
Hexagonal boron nitride structure
In the hot-pressed state, h-BN can be easily machined using conventional metal cutting techniques. Other key properties of h-BN include high dielectric breakdown strength, high volume resistivity, and excellent chemical inertness.
Applications of h-BN
- Hexagonal Boron Nitride is considered very useful in several applications. Here are some of them:
- BN is used as a substrate material for graphene-used electronics. It is used for making dielectrics for future-generation nanoelectronic devices.
- It is used as a high-quality, low-dielectric constant railing material for 2D rampant vertical electron tunneling devices
- Thin h-BN is used for corrosion resistance and antioxidation protective coatings.
- h-BN is effectively used in gas sensing. The material is an essential substance used for sensing gasses, such as ammonia and ethanol.
Cubic Boron Nitride
C-BN is the second hardest material known, second only to diamond. The substance is formed by high-pressure, high-temperature treatment of hexagonal BN. So far, it appears as though carbon and boride do have a lot in common, especially in regards to structure and behavior. While h-BN is analogous to granite, c-BN is similar to and behaves like a diamond — another allotrope of carbon. The properties of cubic boron nitride mirror those of diamond. In the last couple of decades, the application of cubic boron nitride has soared, giving rise to increased commercial production of C-BN.
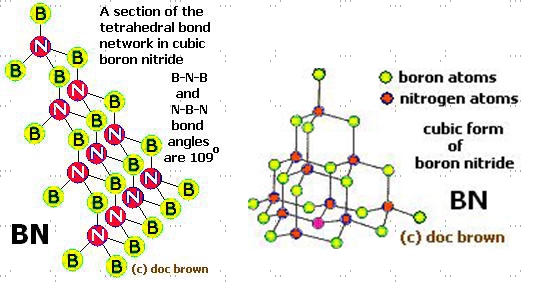
Cubic boron nitride strucure
Regarding its hardness, c-BN is harder than traditional abrasive materials, such as Al2O3, SiC, and boron carbide. This means that the output performance of grinding with c-BN wheels is better than what you get with traditional abrasive material wheels.
Applications of c-BN
- For cutting tools, c-BN is used as sintered c-BN.
- Cubic boron nitride is used as an insulating material in electronics. c-BN is an III-V semiconductor compound. It has a wide band gap, which makes it an excellent insulator.
- c-BN is also known for its excellent wear resistance and inertness to chemical reactions with other reactive materials.
- Because of its high hardness and excellent wear-resistant properties, C-BN is now also used to develop protective coatings.
- The chemical inertness of boron nitride in general makes it suitable as thermocouple protection sheaths, crucibles, and protective linings for reaction vessels.
Conclusion
Boron nitride is an essential ceramic material, existing mainly in three allotropic forms, namely; h-BN, c-BN, and w-BN. While h-BN is attractive for various electronic applications because of its thermal conductive behavior, c-BN serves as an excellent abrasive and machining tool due to its high hardness, which is inferior only to that of the diamond. For more information about ceramic materials, please visit https://www.preciseceramic.com/.
{{item.content}}
LEVE A REPLY
{{item.children[0].content}}
{{item.content}}
LEAVE A REPLY
SUBSCRIBE OUR NEWSLETTER
- How PBN Crucibles Ensure the Quality of GaN & SiC Epitaxial Materials
- SiC vs. Quartz Focus Rings: A Cost and Performance Analysis for Advanced Etch
- AlN Ceramic Substrates: Enabling Next-Gen Electrostatic Chucks
- The Amor of Semiconductor Tools: Why High-Purity Al2O3 & AlN Are Preferred for Plasma Process Chambers
- Silicon Carbide - Ultra-High Temperature Ceramics for Extreme Environments