What is Pyrolytic Boron Nitride (PBN)?

Introduction
Pyrolytic boron nitride is also written as PBN or pyrolytic BN. It is a highly pure ceramic that has high chemical resistance and commendable strength at high temperatures. Boron nitride is a compound made synthetically from boron and nitrogen. This material has good thermal conductivity and is commonly used to make electrical insulators and cutting tools.
Production of Pyrolytic Boron Nitride
As stated earlier, pyrolytic boron nitride is a highly pure ceramic. In some cases, its purity is up to 99.9%. Therefore, it has to be produced under precise conditions. Pyrolytic boron nitride is a type of boron nitride prepared by a pyrolysis reaction at very high temperatures. It is produced by the chemical vapor deposition of boron halides and ammonia. The production process of pyrolytic boron nitride differs from that of hot-pressed boron nitride. One advantage of the chemical vapor deposition process used in pyrolytic boron nitride manufacturing is that the finished product has a uniform layer. As a result of this, pyrolytic boron nitride’s thermal conductivity is anisotropic.
Inter-layer VS. Intra-layer Atomic Spacing in PBN
It can be quite easy to confuse inter-layer atomic spacing and the intra-layer atomic spacing in pyloric boron nitride. However, the two are not the same. The inter-layer atomic spacing is the spacing between atoms in different layers. It is about 3.33 Angstrom. The intra-layer atomic spacing, on the other hand, is the spacing between atoms in the same layer. It is about 1.45 Angstrom.
Pyrolytic Boron Nitride - Hexagonal Structure
Pyrolytic boron nitride has a hexagonal structure. This begs the question, what is a hexagonal boron nitride structure The hexagonal structure is the most stable and softest crystalline form of boron nitride ceramic. Boron nitride with this structure is very similar to graphite. For this reason, it is sometimes referred to as graphitic boron nitride or white graphite. Each layer of the structure has boron and nitrogen atoms. These boron and nitrogen atoms are held together by covalent bonds. The layers within the structure are not held together by covalent bonds. Instead, the layers are bound by van der Waals forces.
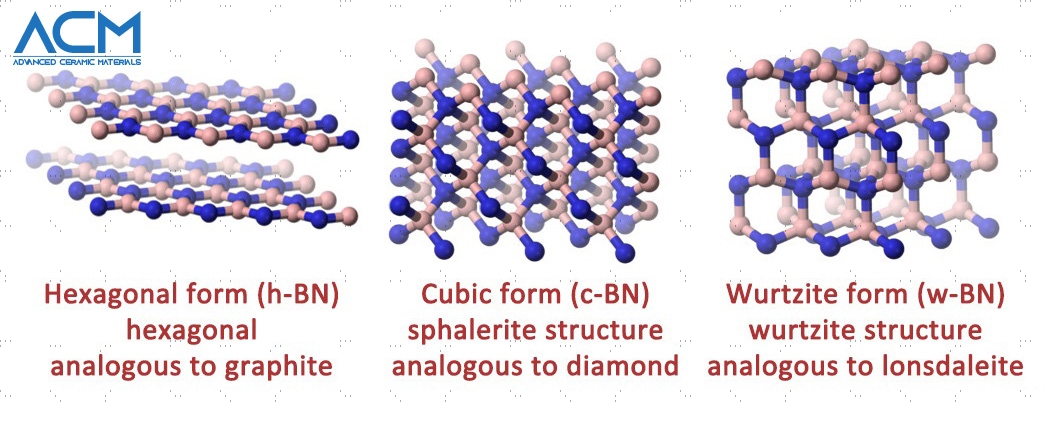
Boron Nitride Structures
Other Boron Nitride Structures
Cubic Boron Nitride Structure
This cubic structure of boron nitride is similar to that of the diamond - the hardest known material in the world. Just as a diamond is less stable than graphite, the cubic structure of boron nitride is less stable than its hexagonal structure. The rings between the layers are in the chair configuration.
Wurtzite Boron Nitride Structure
Just as the cubic boron nitride structure is similar to diamond, the wurtzite boron nitride structure is similar to Lonsdaleite. The boron and nitride atoms in this structure are arranged into six-membered rings. The rings between the layers are in boat configuration It is quite rare to find naturally occurring boron nitride in the wurtzite form. However, they are found in places where there was a volcanic eruption.
Properties of Pyrolytic Boron Nitride
Pyrolytic boron nitride has some properties that distinguish it from other similar materials. These properties could either be physical or chemical.
Physical Properties
Pyrolytic boron nitride has an ivory or orange appearance. In addition, it has a smooth surface that is devoid of pores. Its density is about 2.15 g/cm3. Pyrolytic boron nitride usually has no distinct odor. It is an odorless compound. Furthermore, the structure of pyrolytic boron nitride makes it slippery. It is layered and has a honeycomb appearance. The dielectric strength for pyrolytic boron nitride at room temperature is 56 kV/mm. Also, the tensile strength of this material is 153.84 N/mm2 while its elastic modulus is 235690 N/mm2. The strength of PBN rises with temperature until it gets to 2473 K. At a temperature of 3273 K, pyrolytic boron nitride decomposes into boron and nitride. In addition, it has a satisfactory thermal shock resistance. This means it does not crack easily when there is a sudden temperature change.
Chemical Properties
Pyrolytic boron nitride is a highly pure compound. It is a non-toxic material. At room temperature, this compound does not react with acids, alkalis, or salts. However, it corrodes slightly in lye and it undergoes oxidation at temperatures above 1273 K.
Conclusion
Pyrolytic Boron Nitride is a quality ceramic material with a hexagonal crystal structure. Its high resistance to oxidation and anisotropic thermal conductivity makes it an ideal material for heaters and crucibles. For more information about ceramic materials, please visit https://www.preciseceramic.com/.
{{item.content}}
LEVE A REPLY
{{item.children[0].content}}
{{item.content}}
LEAVE A REPLY
SUBSCRIBE OUR NEWSLETTER
- How PBN Crucibles Ensure the Quality of GaN & SiC Epitaxial Materials
- SiC vs. Quartz Focus Rings: A Cost and Performance Analysis for Advanced Etch
- AlN Ceramic Substrates: Enabling Next-Gen Electrostatic Chucks
- The Amor of Semiconductor Tools: Why High-Purity Al2O3 & AlN Are Preferred for Plasma Process Chambers
- Silicon Carbide - Ultra-High Temperature Ceramics for Extreme Environments