Silicon Carbide (SiC)
Silicon carbide (SiC), or carborundum, is a standout in technical ceramics for its lightweight, exceptionally hard, and strong nature. Initially utilized in the late 19th century for abrasives like sandpapers and grinding wheels, its applications have broadened significantly. Today, SiC is integral to refractory linings, wear-resistant parts in pumps and rocket engines, and as a semiconductor base for LEDs, demonstrating its versatility across both traditional and modern technological applications. This expansion highlights SiC's adaptability and sustained importance in evolving industrial uses, from mechanical engineering to electronics.
The distinctive properties of SiC, such as high strength, thermal shock resistance, and wear resistance, benefit greatly from manufacturing techniques like Pressureless Sintered Silicon Carbide and Reaction Bonded Silicon Carbide. These processes not only enhance SiC's inherent qualities, including chemical resistance and thermal conductivity, but also customize it for specific high-demand applications. Whether ensuring durability in harsh environments or providing mechanical strength, these advanced techniques keep silicon carbide at the forefront of materials needed for high-performance applications in aerospace, semiconductor manufacturing, and beyond, affirming its pivotal role in technological and industrial progress.
More Info About Boron Nitride
Products | Structures | Specification | SiC Types | Applications | Video | FAQs | Safety Data Sheet
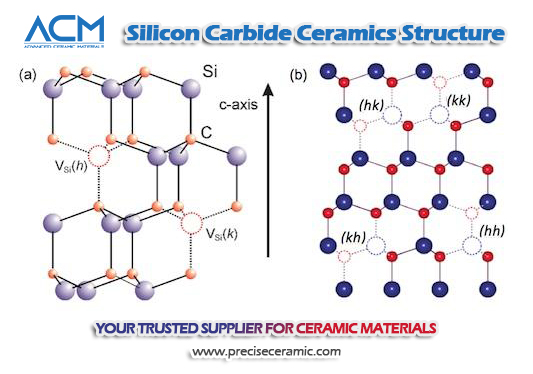
Silicon Carbide Structures
Silicon Carbide (SiC) boasts an extensive array of crystalline forms, with over 250 known polymorphs that illustrate the compound's structural versatility and complexity. These polymorphs share a similar two-dimensional crystal structure yet exhibit variations in their three-dimensional arrangements. This diversity can be visualized as layers meticulously stacked in distinctive sequences, leading to variations in physical properties and, consequently, in their application potential. The ability of SiC to exist in such a multitude of forms underscores its adaptability and the intricate interplay of conditions under which it can be synthesized.
Among the plethora of SiC polymorphs, alpha silicon carbide (α-SiC) and beta silicon carbide (β-SiC) stand out due to their prevalence and significance in industrial applications. α-SiC, recognizable by its hexagonal crystal structure akin to wurtzite, forms at temperatures above 1700°C and is prized for its stability and strength at high temperatures, making it suitable for high-stress applications in harsh environments. On the other hand, β-SiC, with its cubic zinc blende crystal structure, forms at temperatures below 1700°C and is noted for its finer grain size, offering advantages in applications requiring precise detail and high-quality surface finishes. The transition between these polymorphs, influenced by temperature and possibly the presence of catalysts, plays a critical role in tailoring the material's properties for specific uses, ranging from abrasive materials to components in high-power electronic devices.
Silicon Carbide Specification
| Compound Formula | SiC |
| Molecular Weight | 40.1 |
| Appearance | Black |
| Melting Point | 2,730° C (4,946° F) (decomposes) |
| Density | 3.0 to 3.2 g/cm3 |
| Electrical Resistivity | 1 to 4 10x Ω-m |
| Poisson's Ratio | 0.15 to 0.21 |
| Specific Heat | 670 to 1180 J/kg-K |
Silicon Carbide Types
| Attribute | Reaction-bonded Silicon Carbide (RBSC) | Sintered Silicon Carbide (SSC) | Recrystallized Silicon Carbide (RSIC) |
|---|---|---|---|
| Color | Gray to black | Black | Light gray |
| Operating Temperature (°C) | Up to 1400 | Up to 2000 | Up to 2000 |
| Density (g/cm³)** | 3.00 – 3.10 | 3.10 – 3.20 | 2.70 – 2.85 |
| Hardness (Mohs scale) | 9 | 9+ | 9+ |
| Flexural Strength (MPa) | 250 – 300 | 400 – 500 | 150 – 250 |
| Compressive Strength (MPa) | 1800 – 2000 | 2200 – 2500 | 800 – 1200 |
| Thermal Conductivity (W/m·K) | 120 – 140 | 110 – 130 | 60 – 70 |
| Coefficient of Thermal Expansion (10??/°C) | 4.5 – 5.0 | 4.0 – 4.5 | 4.5 – 5.0 |
| Water Absorption (%) | < 0.1 | < 0.1 | 10 – 15 |
| Straightness | High precision, but affected by free silicon | Excellent, precision shapes possible | Good, but porosity can affect precision |
| Process | Mix coarse SiC, silicon, and plasticizers, heat, shape, and machine | Mix fine SiC with sintering aids, heat at 2000°C | Heat pure SiC at 2000°C, recrystallize |
| Applications | Kiln furniture, wear parts, seals, vanes | Seals, pumps, nozzles, bulletproof vests | High-temperature components, thermal applications |
| Advantages | Low cost, easy to machine | High hardness, wear and corrosion resistance | High purity, excellent thermal shock resistance |
| Disadvantages | Contains free silicon, limited to low temps (<1400°C) | Expensive, complex process | High porosity (10-15%) |
Further Reading: 3 Main Production Methods of SIlicon Carbide Ceramics
Silicon Carbide Applications
Silicon Carbide (SiC) is a versatile material with a wide range of applications across various industries, thanks to its exceptional properties of high hardness, thermal conductivity, and thermal shock resistance, among others. Here's an overview of some of the key applications of SiC:
Electronics and Semiconductors
SiC is used in semiconductor electronics that operate at high temperatures or high voltages, or both. With its excellent thermal conductivity and ability to maintain electrical stability under high temperatures, SiC semiconductors are ideal for high-power applications such as power supplies, hybrid vehicles, and high-frequency radio equipment.
Read more: Why is Silicon Carbide Used in Semiconductors
Abrasive and Cutting Tools
Due to its extraordinary hardness, SiC serves as an abrasive in grinding and cutting tools. It is used in the manufacture of grinding wheels, sandpaper, and other abrasive tools, capable of machining metals and materials that would wear down other types of abrasives.
Read more: An Introduction to Silicon Carbide Abrasives
Industrial Furnaces and Heating Elements
The material's high thermal conductivity and resistance to thermal shock make it suitable for use in industrial furnaces and as heating elements. SiC can withstand extreme temperatures and is used in furnaces for sintering, glass production, steelmaking, and other high-temperature processes.
Automotive Applications
SiC is utilized in various automotive applications, including as a material for brakes, clutches, and ceramic parts within the engine. Its durability and resistance to heat make it ideal for high performance and efficiency in automotive components.
Aerospace and Defense
In aerospace and defense, SiC is used in the manufacturing of armor plating and ballistic protective gear due to its high hardness and low density. It provides effective protection against high-velocity projectiles.
Energy
In the energy sector, SiC is used in solar inverters and as a semiconductor in LED lights. Its ability to efficiently convert energy with minimal loss makes it valuable in renewable energy technologies.
Ceramic Matrix Composites
SiC is often used as a reinforcement material in ceramic matrix composites (CMCs). These composites are used in high-temperature applications where conventional metals and alloys would not suffice, such as in jet engines and turbine blades.
Nuclear Applications
Due to its high neutron absorption capability, SiC is considered for use in nuclear reactor fuel pellets and as a material for nuclear waste containment. Its radiation resistance and thermal conductivity are advantageous in managing the heat and radiation levels within nuclear reactors.
Read more: What Are the Uses of Silicon Carbide?
ACM Ceramic Product Categories
Your Silicon Carbide Ceramics Supplier
Advanced Ceramic Materials (ACM) is a leading supplier of silicon carbide ceramic products of the highest quality for a wide range of applications. We are happy to provide advice on materials, design, and application. Feel free to contact us with any questions about SiC or other ceramic materials that are not listed on the website.
| Chemical Formula | SiC |
| Mechanical | |
| Density | 3.22 g/cm3 |
| Hardness | 9.2 Mohs |
| Modulus of Elasticity | 410 GPa |
| Flexural Strength | 550 MPa |
| Compressive Strength | 3.0 GPa |
| Poisson's Ratio | 0.14 |
| Fracture Toughness | 3.0 MPa·m¹/² |
| Electrical | |
| Dielectric Strength | 3.0 x 106 V/cm |
| Dielectric Constant | 9.7 (@ 1 MHz) |
| Volume Resistivity | 104 - 106 ohm·cm |
| Thermal | |
| Coefficient of Thermal Expansion | 4.0 x 10^-6 /°C |
| Thermal Conductivity | 120-270 W/(m*K) |
| Specific Heat | 0.69 J/g·K |
| Shock Resistance | - |
| Maximum Working Temperature | 1650 °C |