An Overview of Boron Carbide Ceramics

Boron carbide (B4C) is a tough ceramic material consisting of boron and carbon. Boron carbide is actually one of the hardest known substances, behind cubic boron nitride and diamond. It is a covalent material used in many important applications, such as tank armor, bulletproof vests, and engine sabotage powders. In fact, it is the material of choice for several other industrial applications. In this article, you’ll get a general overview of what boron carbide is, as well as its benefits.
What is Boron Carbide?
Boron carbide is an important chemical compound with a complex crystal structure typical of icosahedron-based borides. The compound was discovered in the 19th century as a by-product of reactions involving metal borides. Its chemical formula was not known until the 1930s, when its chemical composition was estimated as B4C. X-ray crystallography of the substance shows that its structure is highly complex, with a mixture of C-B-C chains and B12 icosahedra.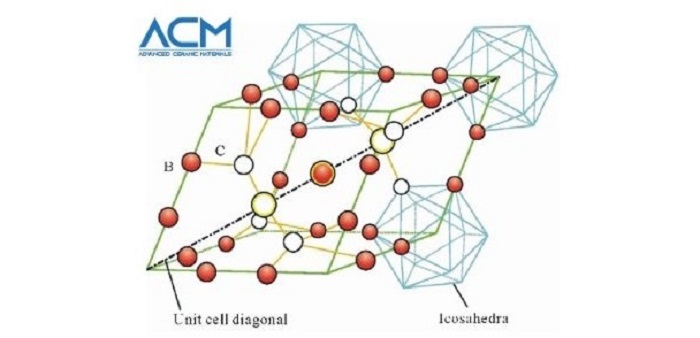
Boron Carbide Crystal Structure
The properties of boron carbide include its extreme hardness (9.5-9.75 on the Mohs hardness scale), stability to ionizing radiation, resistance to chemical reactions, and good shielding properties against neutrons. The Vickers hardness, elastic modulus, and fracture toughness of boron carbide are very close to the corresponding values for diamond. Because of its hardness, boron carbide is also known as black diamond. It has also been shown to exhibit semiconductor properties, with electronic properties dominated by hopping-type transport. It is a p-type semiconductor. Because of its extreme hardness, it is considered a wear-resistant technical ceramic material, making it suited for processing other extremely hard substances. In addition to its good mechanical properties and low specific gravity, it is ideal for making lightweight armor.
Production of Boron Carbide Ceramics
Boron carbide powder can be produced commercially using either fusion (which involves reducing boron anhydride (B2O3) with carbon, or by magnesiothermic reaction (boron anhydride is made to react with magnesium in the presence of carbon black. In the first reaction, the product forms into a sizable egg-shaped lump at the center of the smelter. This egg-shaped material is removed and crushed, and then milled to produce the grain size that is appropriate for final use. In the case of magnesiothermic reaction, stoichiometric carbide with low granularity is obtained directly, but it has impurities, including up to 2% graphite. Because it is a covalently bonded inorganic compound, boron carbide is difficult to sinter without applying heat and pressure simultaneously. Hence, the boron carbide is often preferably hot-pressed into dense shapes using fine and pure powders (<2 μm) under a vacuum or inert atmosphere at high temperatures (2100–2200°C). Another method for producing boron carbide is pressureless sintering at a very high temperature (2300–2400°C), which is close to the melting point of boron carbide. To help in reducing the temperature required for densification during this process, sintering aids like alumina, Cr, Co, Ni, and glass are added to the powder mix.
Applications of Boron Carbide Ceramics
Boron carbide has a variety of applications. Here are some of them: Boron carbide as an abrasive and lapping agent Because of its hardness, boron carbide in the form of powder and pastes is ideally suited for use as an abrasive and lapping agent with a high material removal rate for processing super-hard materials. The application of boron carbide as an abrasive and lapping agent is common in the following fields:
- Boron carbide powders and pastes enable easy surface processing of wear-resistant carbide metals, non-ferrous metals, titanium, ceramics, and hard plastics, such as PTFE.
- Boron carbide pastes are commercially used for the lapping of machine components, machining of tools, drawing dies, wire guides and dies of all kinds, surface treatment of cylinder liners and running surfaces, valves, and valve seats and injection pumps, etc.
Boron carbide is used for ceramic blasting nozzles Due to boron carbide’s exceptional abrasion resistance, it is, in sintered form, an ideal material for blasting nozzles with uniform blasting power, minimal wear, and a longer service life even when used with very hard abrasive blasting agents like corundum and silicon carbide. Boron carbide ceramic is used as a ballistic protective material Compared to armored steel and aluminum oxide, boron carbide produces similar ballistic protection, but at a much lower weight. Modern military equipment depends on a high degree of hardness, compressive strength, and a high modulus of elasticity coupled with low weight. Boron carbide is a better choice compared to all other alternative materials for this purpose.
Further Reading: Application of Boron Carbide Ceramics in Body Armor
Although boron carbide strike face materials behave more glass-like when impacted by high-velocity rounds, it does not affect its superior ballistic performance except against some specific threats where they might underperform.
Conclusion
Boron carbide (B4C) is an extremely powerful abrasive material whose powder is used as starting material for sintering and hot pressing in the manufacture of boron carbide sintered parts. It is the third hardest material known after diamond and cubic boron nitride, which is why it is called black diamond (composed of shiny black crystals with a rhombohedral structure). The material has various industrial applications today. Thank you for reading our article and we hope it can help you to have a better understanding of alumina ceramics. For more information, please visithttps://www.preciseceramic.com/.
{{item.content}}
LEVE A REPLY
{{item.children[0].content}}
{{item.content}}
LEAVE A REPLY
SUBSCRIBE OUR NEWSLETTER
- How PBN Crucibles Ensure the Quality of GaN & SiC Epitaxial Materials
- SiC vs. Quartz Focus Rings: A Cost and Performance Analysis for Advanced Etch
- AlN Ceramic Substrates: Enabling Next-Gen Electrostatic Chucks
- The Amor of Semiconductor Tools: Why High-Purity Al2O3 & AlN Are Preferred for Plasma Process Chambers
- Silicon Carbide - Ultra-High Temperature Ceramics for Extreme Environments