Implication of Boron Nitride: Composites and Applications

Boron nitride ceramic has a chemical and heat-resistant crystalline structure with intractable houses composed of boron and nitride. As it exists in more than one polymorph, BN has advanced as well as an extraordinarily useful compound, locating its purpose in a wide variety of industries and programs.
Properties of Boron Nitride
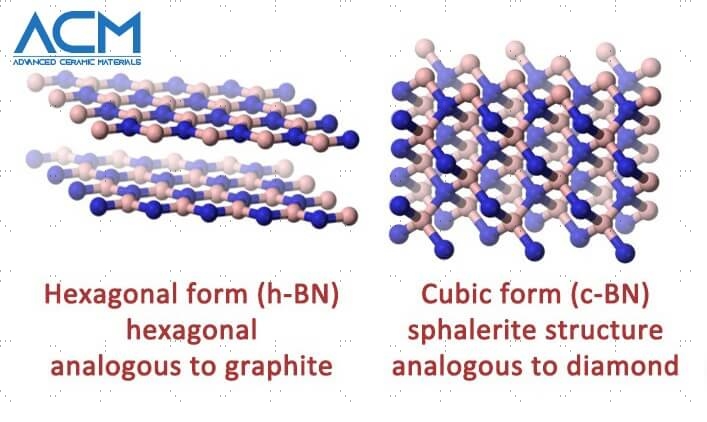
The boron nitride consists of hexagonal structures that appear in crystalline form and is commonly in comparison to graphite. It could come in the form of a leveled lattice or a cubic structure, each of which preserves the chemical and heat resistance that boron nitride is best known for.
-
Heat and chemical resistance
The compound has a melting point of 2,973°c and a thermal enlargement coefficient significantly above that of the diamond. Its hexagonal shape resists decomposition even if exposed to a 1000°c in ambient air. Boron nitride doesn’t dissolve in not unusual acids.
-
Thermal conductivity
At 1700 to 2000 w/mk, boron nitride has a thermal conductivity this is comparable with that of graphene, a similarly hexagon-latticed compound however made up of carbon atoms.
-
lubricating property
Boron nitride has the capacity to reinforce the coefficient of friction of lubricating oil, whilst decreasing the capability for put-on.
-
Density
Depending on its form, its density ranges from 2. 1 to 3.5 g/cm3.
Manufacturing and Processing of Boron Nitride
Boron nitride is synthesized via the response of a boron precursor with a nitrogen-containing reagent underneath a nitrogen atmosphere. This unique chemical reaction yields shapeless boron nitride containing trace amounts of boron trioxide impurities, which can be similarly purified by evaporation thru heating above 1500°c. The flexibility of boron nitride as a compound is evident in the variety of bureaucracy and polymorphs that occur within the actual globe.
Forms of Boron Nitride
-
Hexagonal
This shape of boron nitride has the highest number of programs, because of its excessive lubricating assets, electrical conductivity, and thermal stability.
-
Cubic
The cubic form of BN possesses appreciably high electric resistivity and thermal conductivity like a diamond. It doesn’t dissolve in metallic components, thereby making it a great abrasive fabric.
-
Amorphous
The non-crystalline shape of boron nitride is corresponding to amorphous carbon in terms of structure and residence.
-
Atomically thin
In spite of its ultra-thin property, this BN polymorph is characterized lane of high thermal conductivity, prominent surface adsorption, and suitable dielectric residences.
-
Nanotube
as one of the rising trends nowadays, nanotube generation has been given a lift with the use of boron nitride. This rolled-up form of hexagonal BN is just like carbon nanotubes in terms of shape. But, BN nanotubes have higher electrical insulation in addition to higher resistance to heat and chemical reactions.
Application of Boron Nitride
-
Lubricant
The hexagonal form of boron nitride uses as a lubricant for paints is well known but it is also used in cosmetics, pencil lead, and cement for dental packages. Its lubricating property occurs even within the absence of gas or water molecules within the compound layers, thereby making it a good thing for vacuum structures. As compared to graphite, BN has considerably better chemical balance and electric conductivity.
-
Gadgets in excessive-warmth environments
Its excellent resistance to heat lends the compound to an extensive sort of program regarding extraordinarily high temperatures. Hexagonal boron nitride is getting used to enhance the lubricating residences of rubber, plastic, alloys, and ceramics. In the case of plastics, the inclusion of a BN provides lower thermal growth. It is able to additionally be incorporated into semiconductor substrates and microwave oven home windows. Boron nitride is a powerful factor in response vessels and crucibles due to its thermochemical properties.
-
Semiconductor industry
With a bandgap starting from 4.5 to 6.4 EV, boron nitride is a wonderful huge-hole semiconductor material. Its intrinsic thermal and dielectric properties make it an appropriate substrate in growing metallic-oxide-semiconductor subject-effect transistors and semiconductors.
-
Abrasive and reducing implements
Due to the bodily houses of cubic boron nitride, this polymorph is used as an abrasive material for nickel, iron, and selected alloys in conditions. Wherein diamond became not located be suitable under intense heat. Its cubic BN form is incorporated in reducing-tool bits and grinding gadgets.
![]()
Uses of Boron Nitride Nanoparticles
Boron nitrate nano-structures display unique houses due to their exciting atomic arrangement. Particularly excessive thermal conductivity, chemical inertness, electric resistance, and mechanical energy are of importance in industrial applications.
Boron nitride Nanostructures
Boron nitride (BN) nanostructures are composed of unbalanced boron and nitrate atoms. This connection does not naturally occur in nature as a result they are produced synthetically. BN nanostructures are frequently in comparison to famous carbon nanostructures. That is due to the fact BN is isoelectronic to a carbon lattice and has numerous crystalline forms. There are many feasible utility areas of BN nanostructures because of their favored properties at the nano-scale. Excessive thermal conductivity, mechanical energy, chemical inertness, and insulating residences have the ability to be exploited by using numerous industries. The high thermal conductivity of BN nano-particles is utilized in nano-fluids, Including BN nano-particles to traditional warmness, transfer fluids improve the thermal conductivity and consequently complement the thermal transport. Nano-fluids may be utilized in warmness exchangers for rapid cooling or heating packages. Advanced mechanical electricity of BN nano-particles is used to construct extremely difficult cubic boron nitride (c-BN) with Vickers hardness large than 100 GPA. This fee exceeds the most desirable hardness of artificial diamonds. Further to precise mechanical toughness, c-BN also shows large fracture toughness and excessive oxidation resistance. Due to those precise homes, c-BN may be utilized as a high-overall performance abrasive fabric in the area of a diamond. Furthermore, c-BN is powerful at the precise sculpting of ferrous-based total substances. Boron nitrate is an extensively utilized substance within the industry. With the tendencies in nanotechnology, nanostructures of BN have additionally attracted interest. Specific houses of the hexagonal-BN association are closely exploited in the nanoscale. H-BN primarily based nanostructures display high thermal conductivity, excessive electric resistance, chemical inertness, ultraviolet luminescence, and mechanical strength. BN nanostructures are used for thermal conductivity enhancement, mechanical energy enhancement, insulating coatings, oxidation, and corrosion-resistant coatings, electrical circuits, water remedy, and hydrogen storage. Numerous BN nanostructures together with BN nanotubes and nanoparticles are used to enhance the thermal conductivity of composites and nanofluids. Mechanical electricity of polymer, ceramic, and metal composites is advanced with the aid of the use of BN nanotubes as composite fillers. BN nanotubes and nanoporous BN structures have the ability to be used in hydrogen storage applications due to their high precision surface area. BN nanoparticles are used for acquiring remarkably difficult c-BN substances to particular slicing packages. Despite the fact that commercial programs of BN nanostructures are however in their infancy, there may be exquisite potential for rapid increase.
Conclusion
Thank you for reading our article and we hope it can help you to have a better understanding of boron nitride ceramics. For more information, please visit https://www.preciseceramic.com/.
Reference:
- https://www.intechopen.com/chapters/68044
- https://matmatch.com/learn/material/boron-nitride
- https://nanografi.com/blog/uses-of-boron-nitride-bn-nanopowdernanoparticles/
{{item.content}}
LEVE A REPLY
{{item.children[0].content}}
{{item.content}}
LEAVE A REPLY
SUBSCRIBE OUR NEWSLETTER
- How PBN Crucibles Ensure the Quality of GaN & SiC Epitaxial Materials
- SiC vs. Quartz Focus Rings: A Cost and Performance Analysis for Advanced Etch
- AlN Ceramic Substrates: Enabling Next-Gen Electrostatic Chucks
- The Amor of Semiconductor Tools: Why High-Purity Al2O3 & AlN Are Preferred for Plasma Process Chambers
- Silicon Carbide - Ultra-High Temperature Ceramics for Extreme Environments