How to Use Zirconia in Dentistry?

Ceramic materials are very important in the science of dental biomaterials. Among all dental ceramics, zirconia ceramic is in evidence as a dental biomaterial and it is the material of choice in contemporary restorative dentistry. Zirconia has been applied as a structural material for dental bridges, crowns, inserts, and implants, mostly because of its biocompatibility, high fracture toughness, and radiopacity. However, the clinical success of restorative dentistry has to consider the adhesion to different substrates, which has offered a great challenge to dental zirconia research and development.

The most popular dental ceramic systems are silica-, leucite-, lithium disilicate-, alumina-, and zirconia-based materials. Currently, zirconia-based ceramics are the most studied, challenging researches for different reasons. Zirconia (zirconium dioxide, ZrO2), also named as “ceramic steel”, has optimum properties for dental use: superior toughness, strength, and fatigue resistance, in addition to excellent wear properties and biocompatibility. Zirconium (Zr) is a very strong metal with similar chemical and physical properties to titanium (Ti). Incidentally, Zr and Ti are two metals commonly used in implant dentistry, mostly because they do not inhibit the bone-forming cells (osteoblasts), which are essential for osseointegration.
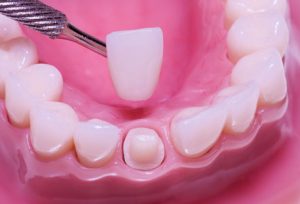
Dental zirconia is, most often, a modified yttria (Y2O3) tetragonal zirconia polycrystal (Y-TZP). Yttria is added to stabilize the crystal structure transformation during firing at an elevated temperature and improve the physical properties of zirconia. Upon heating, the monoclinic phase of zirconia starts transforming to the tetragonal phase at 1187 °C, peaks at 1197 °C, and finishes at 1206 °C. On cooling, the transformation from the tetragonal to the monoclinic phase starts at 1052 °C, peaks at 1048 °C, and finishes at 1020 °C, exhibiting a hysteresis behavior. The zirconia tetragonal-to-monoclinic phase transformation is known to be a martensitic transformation.
{{item.content}}
LEVE A REPLY
{{item.children[0].content}}
{{item.content}}
LEAVE A REPLY
SUBSCRIBE OUR NEWSLETTER
- How PBN Crucibles Ensure the Quality of GaN & SiC Epitaxial Materials
- SiC vs. Quartz Focus Rings: A Cost and Performance Analysis for Advanced Etch
- AlN Ceramic Substrates: Enabling Next-Gen Electrostatic Chucks
- The Amor of Semiconductor Tools: Why High-Purity Al2O3 & AlN Are Preferred for Plasma Process Chambers
- Silicon Carbide - Ultra-High Temperature Ceramics for Extreme Environments