Boron Carbide (B4C)
Boron Carbide (B4C) is distinguished by its extreme hardness, rated between 9 and 10 on the Mohs scale, making it one of the hardest synthetic substances, exceeded only by cubic boron nitride and diamond. This covalent ceramic is utilized in high-stakes applications such as tank armor, bulletproof vests, and industrial uses like engine sabotage powders due to its excellent chemical inertness and significant neutron absorption capacity. Its use extends to antioxidant components in refractory mixes, highlighting its versatility in advanced technological applications.
Despite its remarkable hardness and brittleness, Boron Carbide's low thermal conductivity and susceptibility to thermal shock do not compromise its utility in demanding environments. Additionally, its properties as a semiconductor underline its importance in semiconductor technology. Boron Carbide's unique blend of physical and chemical characteristics ensures its pivotal role in applications requiring materials that perform reliably under extreme conditions.
More Info About Boron Carbide
Products | Structure | Specification | Material Grades | Applications | Video | FAQs | Safety Data Sheet | Product Manual
Boron Carbide Ceramics Structure
Boron Carbide (B4C) features a unique crystal structure that contributes to its exceptional hardness and other remarkable properties. Its structure is rhombohedral, and the material is composed of a complex lattice of boron and carbon atoms. The crystal lattice can be described as consisting of icosahedra of boron atoms with carbon atoms positioned in the interstices, creating a structure that is both dense and covalently bonded.
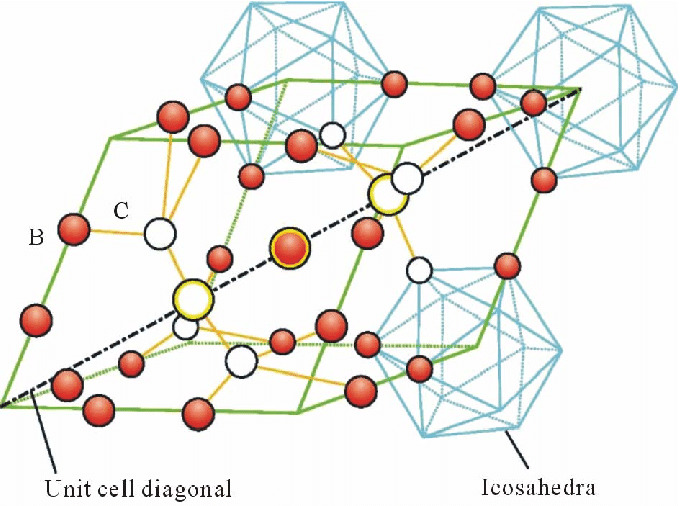
In the boron carbide structure, the boron atoms form 12-atom icosahedra which are linked by the carbon atoms. These carbon atoms act as bridges between the boron icosahedra, contributing to the material's extreme hardness and resistance to deformation. The B4C crystal structure is characterized by a combination of strong covalent bonds within the icosahedra and between them, providing the material with its high hardness, which is only slightly less than that of diamond and cubic boron nitride.
This structural arrangement also results in several other significant properties of boron carbide, such as its low density compared to other ceramics, making it valuable for lightweight armor applications. Additionally, the structure imparts good chemical inertness, allowing it to resist attack by most acids and alkalis. The complex interplay of boron and carbon atoms within the boron carbide lattice is key to the material's impressive performance across a range of demanding applications.
Boron Carbide Ceramics Specification
|
Properties |
Units |
Value |
|
Chemical Formula |
|
B4C |
|
Density |
gm/cm3 |
2.1 to 2.7 |
|
Melting Point |
℃ |
2445 |
|
Hardness (Knoop) |
kg/mm |
2800 |
|
Flexural Strength 4pt |
Mpa |
425 |
|
Compressive Strength |
Mpa |
2900 |
|
Modulus of Elasticity |
Gpa |
440 |
|
Poisson's Ratio |
|
0.17 to 0.18 |
|
Specific Heat |
J/kg-K |
950 |
|
Fracture Toughness |
Mpa x m1/2 |
3.1 |
|
Coefficient of Thermal Expansion |
x10-6 mm/mmK |
5 |
|
Thermal Conductivity |
W/mK |
31 to 90 |
Different Grades of Boron Carbide
| Property | Unit | B4C | B4C +SiC | SiC+B4C) |
|---|---|---|---|---|
| Density | g/cm3 | 2.52 | 2.62 | 2.8 |
| Hardness | GPa | 33 | 31 | 28 |
| Fracture Toughness KIc 1 | MPa m2 | 2.5 | 3.4 | 4 |
| Compressive Strength | MPa | 3000 | 2900 | 2500 |
| Flexural Strength @ 25°C | MPa | 420 | 340 | 330 |
Boron Carbide Applications
Boron Carbide (B4C) is a versatile material known for its extreme hardness and durability, making it suitable for a wide range of applications.
Armor
Body Armor and Protective Gear: Leveraging its incredible hardness and lightweight nature, B4C is extensively used in the production of body armor, helmets, and vehicle armor. Its ability to absorb and disperse high-impact energies makes it vital for personal protection in law enforcement.
Read more: Application of Boron Carbide Ceramics in Body Armor
Industrial Uses
Abrasives: Boron carbide's hardness makes it ideal for use as an abrasive in water jet cutting, as well as in the manufacture of grinding wheels and abrasive powders. Its wear resistance ensures efficiency and longevity in cutting and grinding operations.
Nozzles: In industrial applications requiring the handling of abrasive materials, B4C nozzles offer superior durability and resistance to wear, particularly in sandblasting and spray nozzle applications.
Semiconductor Industry
Ion Implantation: In the semiconductor manufacturing process, boron carbide is used as a material for ion implantation. Its properties facilitate the doping of semiconductors with boron ions, a step crucial for modifying electrical properties in semiconductor devices.
Advanced Engineering Materials
Refractory Components: The chemical inertness and thermal stability of B4C make it suitable for use in refractory applications. Components made from boron carbide can withstand high temperatures and aggressive environments, making them ideal for furnaces and kilns.
Read more: Boron Carbide: Key Properties & Applications
ACM Ceramic Product Categories
Your Boron Carbide Ceramics Supplier
Advanced Ceramic Materials (ACM) is a leading supplier of boron carbide ceramic products of the highest quality for a wide range of applications. We are happy to provide advice on materials, design, and application. Feel free to contact us for any questions about B4C or other ceramic materials that are not listed on the website.
Read more: An Overview of Boron Carbide Ceramics
| Chemical Formula | B4C |
| Mechanical | |
| Density | 2.52 g/cm3 |
| Hardness | 27.4 GPa |
| Modulus of Elasticity | 450 GPa |
| Flexural Strength | 350 MPa |
| Compressive Strength | 3000 MPa |
| Poisson's Ratio | 0.2 |
| Fracture Toughness | 3.2 MPa m½ |
| Thermal | |
| Coefficient of Thermal Expansion | 2.7 x 10^-6/°C |
| Thermal Conductivity | 27 W/(m*K) |
| Specific Heat | 1854 x10^3 J/(Kg*K) |
| Shock Resistance | - |
| Maximum Working Temperature | 600 °C |